Answer:
The percent error of the density calculation is 2.2872%
Step-by-step explanation:
Accepted value for the density of iron = 7.87 g/cm3
Mass = 153.8 g
Volume = 20 cm3
Percent error?
Percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or accepted value. It is often used in science to report the difference between experimental values and expected values.
The formular is given below as;
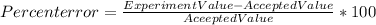
Steps to Calculate the Percent Error
- Subtract the accepted value from the experimental value.
- Take the absolute value of step 1
- Divide that answer by the accepted value.
- Multiply that answer by 100 and add the % symbol to express the answer as a percentage.
Density = Mass / Volume = 153.8 / 20 = 7.69g/cm3
Experiment Value = 7.69g/cm3
Step 1
Experiment Value - Accepted Value = 7.69 - 7.87 = -0.18
Step 2
|-0.18 g/cm3| = 0.18 g/cm3
Step 3
0.18 / 7.87 = 0.02287
Step 4
0.02287 * 100 = 2.2872%
The percent error of the density calculation is 2.2872%.