Question
What does your gauge read?
Answer:
214.576 kPa
Step-by-step explanation:
Denver’s temperature in Kelvin= 10+273=283 K
Fairplay’s temperature in Kelvin= 0+273= 273 K
To get absolute pressure, we add atmospheric pressure to gauge pressure
Absolute pressure at Denver=85 +210=295 kPa
Absolute pressure at Fairplay= 70+ Gauge pressure
We know that from ideal gas equation, pressure is directly proportional to temperature hence
where the subscripts D and F denote Denver and Fairly respectively, P and T denote pressure and temperature respectively
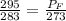
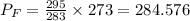
Since =70+Gauge pressure, therefore, gauge pressure=284.576-70=214.576 kPa