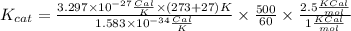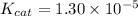Answer:
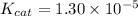
Step-by-step explanation:
turnover number for an enzyme = 500/min
destabilization of the ES complex = 1Kcal/mole
destabilization of transition state (ES‡ ) = 2.5 kcal/mol
kB = 3.297×10−24 cal/K
h = 1.583 x 10-34 cal.s
R = 2 cal/K.mol
we Know that
kcat = (kBT/h).e-(ΔG‡/RT)
T = temperature in Kelvin
By considering the following reaction
K1 K2
E + S ⇆ (ES‡ ) ⇒ E + P
K-1
Ф = K2(ES‡ )
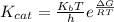
where
ΔG‡ = -RTlnKeq‡
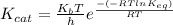
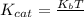 K‡eq
K‡eq
K(cat)= Kb T/h x K2x ES‡/ES Now by putting values we get