Answer:
0.1127 grams of Mg is used to produce one gram of silver
Step-by-step explanation:
Write a balanced equation
Mg(s) +2Ag + (aq) -->
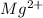 (aq) + 2Ag(s)
(aq) + 2Ag(s)
The atomic mass of magnesium is 24.31 g/mol and the atomic mass of silver is 107.84g/mol
1 g Ag of silver is produced from [(1 mol Mg)(24.31 g/mol)] /[ (2mol Ag)(107.84g/mol)]
1 g Ag of silver is produced from 0.1127 grams of Mg
0.1127 grams of Mg is used to produce one gram of silver