Answer:
Average atomic mass = 10.812 amu
Step-by-step explanation:
The formula for the calculation of the average atomic mass is:
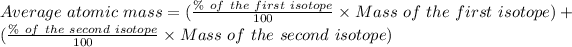
Given that:
For first isotope, Boron-10:
% = 19.8 %
Mass = 10.013 amu
For second isotope, Boron-11:
% = 80.2 %
Mass = 11.009 amu
Thus,
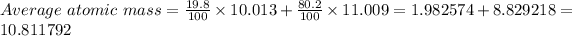
Average atomic mass = 10.812 amu