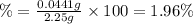Answer:
The mass percentage of nitrogen in the sample is 1.96%.
Step-by-step explanation:
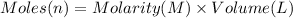
21.0 mL of 0.150 M HCl
Moles of hydrogen chloride = n
Volume of hydrogen chloride solution = 21.0 mL = 0.021 L
Molarity of the hydrogen chloride = 0.150 M
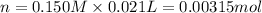
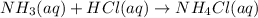
According to recation, 1 mole of HCl neutralizes 1 mole ammonia.Then 0.00315 moles of HCl will neutrtalize:
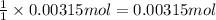 of ammonia
of ammonia
1 mole of ammonia has 1 mole of nitrogen atom . Then 0.00315 moles of ammonia will have = 1 × 0.00315 mol = 0.00135 mol
Mass of 0.00315 moles of nitrogen= 0.00315 mol × 14 g/mol = 0.0441 g
Mass of the sample = 2.25 g
The mass percentage of nitrogen in the sample: