Answer:
0.311 M
Step-by-step explanation:
HA, our weak acid, reacts with NaOH by the following equation given below:
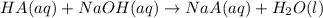
This may also be represented by the net ionic equation for this acid-base reaction:
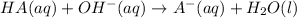
During the titration up to the equivalence point, we would have some HA remaining and some
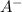 produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:
produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:
![pH = pK_a + log(([A^-])/([HA))](https://img.qammunity.org/2020/formulas/chemistry/college/8zef92e0508fwcbuz2tunciaw924lw6bp3.png)
At midpoint, half of HA reacts to produce half of
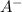 , meaning
, meaning
![[HA] = [A^-]](https://img.qammunity.org/2020/formulas/chemistry/college/94w71cqhu7tj1333fbo47xgxovcn3b2jnb.png) when half of equivalence volume of NaOH is added.
when half of equivalence volume of NaOH is added.
This implies that:
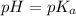 at midpoint. We may then find the acid ionization constant for this acid:
at midpoint. We may then find the acid ionization constant for this acid:
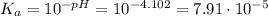
The initial molarity has to be found now. We know that the initial pH is:
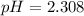
Since
![pH = -log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ced4ktntln1flx5tyce2xmc2f2fst29mie.png) , then
, then
![[H_3O^+] = 10^(-pH)](https://img.qammunity.org/2020/formulas/chemistry/college/pvvwf7b912gda2vk4ekhq4q55i62pweajy.png)
Using an ICE table, for some initial molarity of acid,
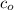 , we may write the equilibrium constant expression as:
, we may write the equilibrium constant expression as:
![K_a = ([H_3O^+]^2)/(c_o - [H_3O^+])](https://img.qammunity.org/2020/formulas/chemistry/college/xsbn1xyapa1vgyac8ij29h2xfm7scnc1e4.png)
Solve for the initial molarity of HA:
![c_o = ([H_3O^+]^2)/(K_a) + [H_3O^+] = (10^(-2pH))/(K_a) + 10^(-pH) = (10^(-2\cdot 2.308))/(7.91\cdot 10^(-5)) + 10^(-2.308) = 0.311 M](https://img.qammunity.org/2020/formulas/chemistry/college/m9xy0gv9m0h465eoz9nxstm6vh797m86a4.png)