Answer:
4
Step-by-step explanation:
Avogadro’s number represent the number of the constituent particles which are present in one mole of the substance. It is named after scientist Amedeo Avogadro and is denoted by
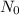 .
.
Also, it is the number of particles in exactly 12.000 g of isotope carbon 12.
Avogadro constant:-
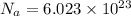
Hence,
Mass of
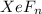 = 131.293+ n18.998 g
= 131.293+ n18.998 g
So,
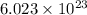 molecules have a mass of 131.293+ n18.998 g
molecules have a mass of 131.293+ n18.998 g
Also,
 molecules have a mass of
molecules have a mass of
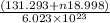 g
g
So,
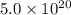 molecules have a mass of
molecules have a mass of
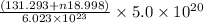 g
g
Also, given mass = 0.172 g
Thus,
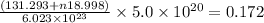
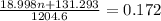
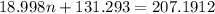
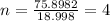
Thus, value of n is 4.