Answer:
the height of the isopropyl iodide column in the barometer in cm =578.208 cm
Step-by-step explanation:
Pressure of the liquid column=ρgh
ρ= density of the liquid
h= height of the column
g= acc. due to gravity
height of the mercury column corresponding to 0.951 atm
= 0.951×76/1atm = 72.276 cm
since, pressure to be measured is same
1= mercury
2=isopropyl iodide
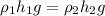
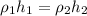
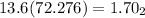
h2= 578.208 cm
the height of the isopropyl iodide column in the barometer in cm =578.208 cm