Answer:
11.03 moles of photons, with wavelength of 12 cm, must be absorbed to raise the temperature of your eye by 3.0 ºC.
Step-by-step explanation:
Mass of eye = m = 11 g
The specific heat capacity = c = 4.0 J/g°C
Change in temperature = ΔT = 3.0°C
Heat required to raise the temperature = Q
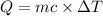
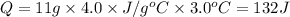
Energy of the photon = E
Wavelength of the photon
 = 12 cm = 0.12 m (1 cm = 0.01 m)
= 12 cm = 0.12 m (1 cm = 0.01 m)
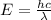 (Planck's equation)
(Planck's equation)
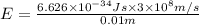
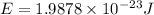
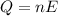
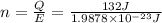
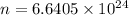
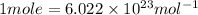
Moles of photons :
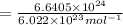
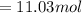
11.03 moles of photons, with wavelength of 12 cm, must be absorbed to raise the temperature of your eye by 3.0 ºC.