Answer: The correct answer is option A.
![[PO_4^(3-)]<[NO_3^(-)]<[Na^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/1ysj0h7rxflhmb7ydvq2g6crosw55lt4s2.png)
Step-by-step explanation:
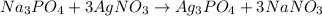
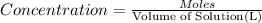
100 mL of 1.0 M
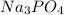
Volume of
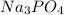 = 100 mL = 0.1 L
= 100 mL = 0.1 L
Moles of
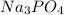 = n
= n
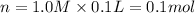
1 mole of
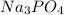 gives 3 moles of sodium ions and 1 mole of phosphate ions.
gives 3 moles of sodium ions and 1 mole of phosphate ions.
Moles of sodium ions =
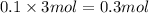
Volume of solution after mixing = 200 mL = 0.2 L
Concentration of sodium ions=
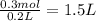
100 mL of 1.0 M

Volume of
 = 100 mL = 0.1 L
= 100 mL = 0.1 L
Moles of
 = n'
= n'
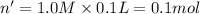
1 mole of
 gives 1 mole of silver ions and 1 mole of nitrate ions.
gives 1 mole of silver ions and 1 mole of nitrate ions.
According to reaction 1 mole of
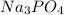 reacts with 3 moles of
reacts with 3 moles of
 .
.
Then 0.1 mole of
 will react with:
will react with:
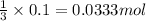 of
of
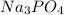
Moles of sodium phosphate left unreacted = 0.1 mol - 0.0333 mol = 0.0667 mol
1 mole of
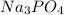 gives 3 moles of sodium ions and 1 mole of phosphate ions.
gives 3 moles of sodium ions and 1 mole of phosphate ions.
As we can see that silver nitrate is in limiting amount
According to reaction 3 mole of
 gives with 1 mole of
gives with 1 mole of
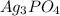 .
.
So, when 0.1 mol of
 reacts it gives:
reacts it gives:
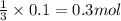 of
of
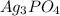
Moles of phosphate ions left in solution=
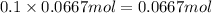
Volume of solution after mixing = 200 mL = 0.2 L
Concentration of phosphate ions=
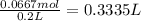
Moles of nitrate ions =
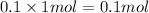
Volume of solution after mixing = 200 mL = 0.2 L
Concentration of nitrate ions=
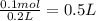
But is an excessive reagent its concentration will be less
![[PO_4^(3-)]<[NO_3^(-)]<[Na^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/1ysj0h7rxflhmb7ydvq2g6crosw55lt4s2.png)