Answer:
it should be increased by 220.73K or -52.22Celsius
Step-by-step explanation:
A Carnot engine has a lower operating temperature of 14.6 Celsius and efficiency of 21.2 %. By how many kelvins should the high operating temperature be increased to achieve an efficiency of 50.9 %?
effi=
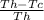
Tc=temperature of the cold reservoir in kelvin(14.6+273=287.6
Th=temperature of the hot reservoir
eff= efficiency of a carnot engine(which alway <100%)
effi=
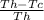
21.2 %=1-Tc/Th
0.212=1-287.6/Th
-.788=-287.6/Th
Th=364.97K
to achieve an efficiency of 50.9%
0.509=1-Tc/Th
-0.491=-287.6/Th
Th=585.74K
taking the difference between the two high temperatures
585.74K-364.97K
220.73K
it should be increased by 220.73K