Answer:
- 0.07 °C
Step-by-step explanation:
At constant pressure and number of moles, Using Charle's law
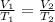
Given ,
V₁ = 439 mL = 0.439 L ( 1 L = 0.001 mL )
V₂ = 0.378 L
T₁ = 317.15 K
T₂ = ?
Using above equation as:
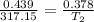
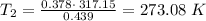
The conversion of T(K) to T( °C) is shown below:
T( °C) = T(K) - 273.15
So, T = 273.08 - 273.15 °C = - 0.07 °C