Answer:
The height is 0.247 m.
Step-by-step explanation:
Given that,
Spring constant = 1.60\times10^3\ N/m[/tex]
Pressure = 1.00 atm
Temperature = 20.0°C
Suppose if the piston has a cross section area of 0.0120 m² and negligible mass, how high will it rise when the temperature is raised to 250°C
The equation of the pressure is
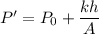 ...(I)
...(I)
The equation of the volume is
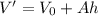 ....(II)
....(II)
We need to calculate the height
Using equation of ideal gas
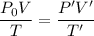
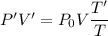
Put the value of P' and V' from equation (I) and (II)
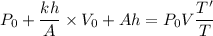
Put the value into the formula
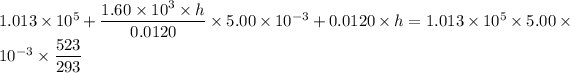
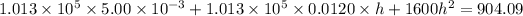
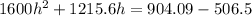
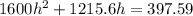
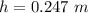
Hence, The height is 0.247 m.