There are 11 Carbon atoms in the compound.
Solution:
Carbon atom count is the ratio of the M peak to the M+1 peak.
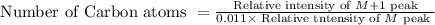
Here M peak is 57.10% and M+1 peak is 6.83%. On applying the values in the formula we get,
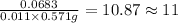
Therefore, the number of Carbon atoms in the compound are 11.
Refer the image attached below for a better understanding of M peak and M+1 peak.
The heaviest ion that has the greatest m/z value is said to be the molecular ion peak in mass spectrum.