Answer:
i.e. 393650 kg.
Explanation:
Given that Radium-226 is a radioactive substance with an annual decay rate of 0.04 %
Let the year 1937 be 0 year
Initially population was = 400,000 kg
Since decay rate is 0.04% per year after t years population would be
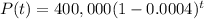
=
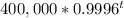
In 2017, t = 40 years (i.e. years lapsed form 1937)
Hence population
=
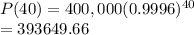
i.e. 393650 kg.