Answer:
The two gases are
 and
and
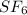
Step-by-step explanation:
According to Graham law of effusion for a binary mixture of gases-
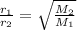
Where
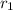 and
and
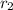 are rate of effusion of gas-1 and gas-2 respectively.
are rate of effusion of gas-1 and gas-2 respectively.
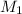 and
and
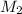 are molar mass of gas-1 and gas-2 respectively.
are molar mass of gas-1 and gas-2 respectively.
Gas Molar mass (g/mol)
 32
32
 71
71
CO 28
 44
44
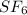 146
146
So, clearly molar mass of
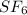 is greater than molar mass of
is greater than molar mass of
 . Hence
. Hence
 will effuse faster.
will effuse faster.
Also,
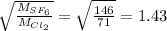
Hence, option (d) is correct.