Answer:
(a)
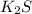 (b)
(b)
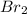
Step-by-step explanation:
(a)
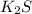 is an ionic compound in which
is an ionic compound in which
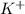 and
and
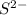 ions are tightly bound together through ion-ion attraction force (principal intermolecular force).
ions are tightly bound together through ion-ion attraction force (principal intermolecular force).
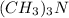 is a polar covalent molecule. So dipole-dipole attraction force (principal intermolecular force) exists in this molecule.
is a polar covalent molecule. So dipole-dipole attraction force (principal intermolecular force) exists in this molecule.
We know, ion-ion attraction force is stronger than dipole-dipole attraction force. Therefore more energy is required to boil liquid
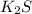 by disrupting stronger ion-ion attraction force as compared to
by disrupting stronger ion-ion attraction force as compared to
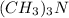 .
.
So,
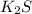 has higher boiling point.
has higher boiling point.
(b) Both
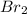 and
and
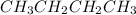 are nonpolar covalent compounds. Hence only london dispersion force(principal intermolecular force) exists in both molecules.
are nonpolar covalent compounds. Hence only london dispersion force(principal intermolecular force) exists in both molecules.
London dispersion force is proportional to molas mass of a molecule.
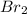 has higher molar mass as compared to
has higher molar mass as compared to
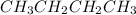 . Therefore
. Therefore
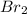 has higher boiling point.
has higher boiling point.