Answer:
Methanol is more acidic than the alkyne and will be deprotonated instead.
Step-by-step explanation:
 of methanol is around 15 and
of methanol is around 15 and
 of terminal alkyne is around 26.
of terminal alkyne is around 26.
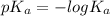 , where
, where
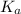 is acid dissociation constant
is acid dissociation constant
So, higher the acidity of an acid, higher will its
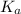 value and thereby lower will be its
value and thereby lower will be its
 value.
value.
So, methanol is certainly stronger acid than terminal alkyne.
Hence sodium amide preferably deprotonates methanol instead of terminal alkyne.
Hence, option (A) is correct.