Answer:
n = 6
Step-by-step explanation:
The processes of multifontic absorption various photons are absorbed simultaneously
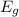 = n E₀
= n E₀
Where Eg is the energy gap, E₀ is the energy of each photon and n is an integer.
In our case, let's calculate the photon's energy using Planck's relationship
E = h f
The speed of light is related to wavelength and frequency
c = λ f
f = c / λ
We replace
E = h c /λ
Let's calculate
E₀ = 6.63 10⁻³⁴ 3 10⁸/800 10⁻⁹
E₀ = 2,486 10⁻¹⁹ J
We reduce to eV, they are easier to use
E₀ = 2,486 10⁻¹⁹ J (1 eV / 1.6 10⁻¹⁹ J)
E₀ = 1.55 eV
We clear from the first equation
n =
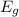 / Eo
/ Eo
n = 9.3 / 1.55
n = 6
Six photons need to be absorbed at the same time