Step-by-step explanation:
The dissociation of lithium bromide is given by the thermochemical equation as,
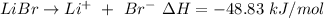
Number of moles, n = 2 moles
Molar mass of Lithium bromide is 86.845 g/mol
For the two moles, the heat released is,
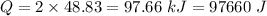
For 2 moles of Lithium Bromide,
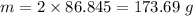
Initial temperature,
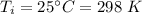
The heat capacity is given by :
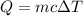

 is the final temperature
is the final temperature

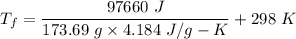
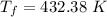
So, the final temperature of the water is 432.38 k. Hence, this is the required solution.