Answer:
P = 0.68 atm.
Step-by-step explanation:
Assuming that we can treat the helium as an ideal gas, we can apply the ideal gas equation to both states, as follows:
Initial State:
P₁*V₁ = n*R*T₁
Final State:
P₂*V₂ = n*R*T₂
Dividing both sides, and arranging terms, we get:
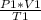 =
=
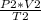
Replacing by the givens, we can solve for P₂:
P₂ = 331.15 K * 2.00 L * 15.18 psi / (310.15 K * 3.24 L) = 10.00 psi
As the result is requested in atm, we need to convert from psi (pound/sq in) to atm, as follows:
P(atm) = 10.00 psi* 1/14.696 atm/psi = 0.68 atm