Step-by-step explanation:
Formula for the first order decay is as follows.
 = -kt
= -kt
where, A = activity at time t
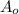 = initial activity
= initial activity
k = decay constant
Hence, putting the given values into the above formula as follows.
k =
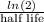
=
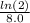
= 0.086643 per day
Also,
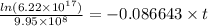
t = 32 days
Thus, we can conclude that it will take 32 days for the activity to drop to

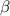 particles per day.
particles per day.