Answer:
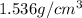 is the density of solid calcium.
is the density of solid calcium.
Step-by-step explanation:
Formula used :
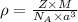
where,
 = density
= density
Z = number of atom in unit cell
M = atomic mass
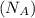 = Avogadro's number
= Avogadro's number
a = edge length of unit cell
We have:
r = 197 pm =
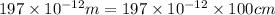
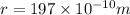
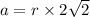
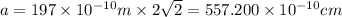
M = 40 g/mol
Z = 4
On substituting all the given values , we will get the value of 'a'.
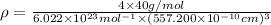
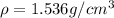
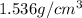 is the density of solid calcium.
is the density of solid calcium.