Answer:
Δn = 2.25 moles
Step-by-step explanation:
given,
n₁ = 3 mol
initial volume = V
final volume = V/4
temperature is constant = ?
using ideal gas equation
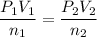
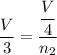
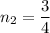
n₂ = 0.75 moles
moles of gas allowed to escape
Δn = n₁ - n₂
Δn = 3 - 0.75
Δn = 2.25 moles
moles of gas allowed to escape is equal to Δn = 2.25 moles