Answer:
The volume at the surface is 10.97 L.
Step-by-step explanation:
Given that,
Volume = 5.5 L
Height = 10 m
Density of sea water= 1025 kg/m³
We need to calculate the pressure at that point
Using formula of pressure
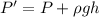
Put the value into the formula
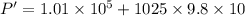
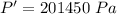
We need to calculate the volume at the surface
Using equation of ideal gas
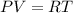
So, for both condition
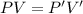
Put the value into the formula
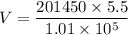
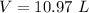
Hence, The volume at the surface is 10.97 L.