Answer:
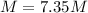
Step-by-step explanation:
Hello,
In this case, the cocaine hydrochloride whose molecular formula is:
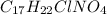
Has a molar mass of 339.8 g/mol, for that reason, in 1.00 g there are the following moles:
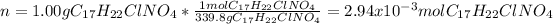
Such calculations are performed at the saturation condition with which the molarity is obtained as:
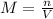
Thus, the volume in liters is:
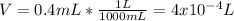
As we assume the volume does not change when the cocaine hydrochloride is added to the water, therefore, we obtain the molarity:
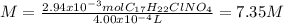
Best regards.