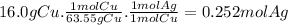Answer:
0.252 mol
Step-by-step explanation:
Given the following reaction:
Cu + 2 AgNO₃ → 2 Ag + Cu(NO₃)₂
How many moles of Ag will be produced from 16.0 g Cu, assuming AgNO3 is available in excess.
First, we write the balanced equation.
Cu + 2 AgNO₃ → 2 Ag + Cu(NO₃)₂
We can establish the following relations.
- The molar mass of Cu is 63.55 g/mol.
- The molar ratio of Cu to Ag is 1:1.
The moles of Ag produced from 16.0 g of Cu are: