Answer:
(1)NBS, ROOR (2)
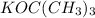 and (3)HBr, ROOR
and (3)HBr, ROOR
Step-by-step explanation:
Conversion of ethylbenzene to (2-bromoethyl)benzene involves three steps.
First step: allylic bromination of ethylbenzene in presence of NBS, ROOR. This step produces (1-bromoethyl)benzene.
Second step: E2 ellimination of HBr from (1-bromoethyl)benzene in presence of a strong base e.g. potassium t-butoxide [
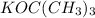 ]. This step produces styrene
]. This step produces styrene
Third step: HBr addition to double bond in presence of HBr, ROOR (antimarkonikov addition of HBr). Thus step produces (2-bromoethyl)benzene.
Full reaction scheme has been shown below.