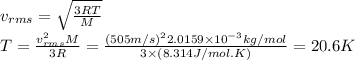Answer:
20.6 K
Step-by-step explanation:
The root mean square velocity is the square root of the average of the square of the velocity. It can be calculated using the following expression.
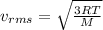
where,
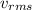 : root mean square velocity
: root mean square velocity
R: ideal gas constant
T: absolute temperature
M: molar mass
Then, we can find the temperature,