Answer:
Whether barium chloride solution was pure
Step-by-step explanation:
We may answer whether barium chloride was pure. The sequence of this experiment might be depicted by the following balanced chemical equations:
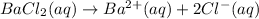
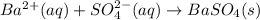
Having a total sample of 10.0 grams, we would firstly find the mass percentage of barium in barium chloride:
![\omega_(Ba^(2+)) = (M_(Ba))/(M_(BaCl_2)) = \frac{137.327 g/mol}{208.23 g/mol\cdot 100\% = 65.95 \%]()
This means in 10.0 g, we have a total of:
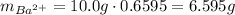 of barium cations.
of barium cations.
The precipitate is then formed and we measure its mass. Having its mass determined, we'll firstly find the percentage of barium in barium sulfate using the same approach:
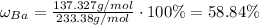
Multiplying the mass we obtained by the fraction of barium will yield mass of barium in barium sulfate. Then:
- if this number is equal to 6.595 g, we have a pure sample of barium chloride;
- if this number is lower than 6.595 g, this means we have an impure sample of barium chloride, as we were only able to precipitate a fraction of 6.595 g.