Answer:
2.83 g
Step-by-step explanation:
At constant temperature and pressure, Using Avogadro's law
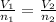
Given ,
V₁ = 2.12 L
V₂ = 3.12 L
n₁ = 0.120 moles
n₂ = ?
Using above equation as:
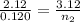
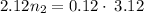
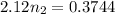
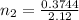
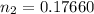
n₂ = 0.17660 moles
Molar mass of methane gas = 16.05 g/mol
So, Mass = Moles*Molar mass = 0.17660 * 16.05 g = 2.83 g
2.83 g are in the piston.