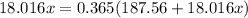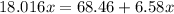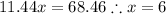Answer:
6
Step-by-step explanation:
The percent mass is equal to the ratio between the mass of the component of interest and the total mass of the compound, multiplied by 100 % in case we need to express it as a percentage.
We may apply this knowledge to write the formula for copper(II) nitrate x-hydrate. Let's assume that we take 1 mole of the compound. Then we will divide x molar masses of water by the molar mass of the overall compound and multiply by 100 % to get the mass percent of water:
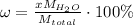
Here:
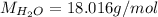
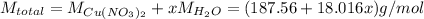
Substitute into the formula:
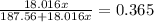
Solve for x: