Answer: Option (B) is the correct answer.
Step-by-step explanation:
Equilibrium constant is defined as the relationship present between the amounts of products and reactants which are there at equilibrium in a reversible chemical reaction at a given temperature.
For example,
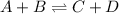
Mathematically,
![K_(eq) = [C][D]](https://img.qammunity.org/2020/formulas/chemistry/high-school/vdydomstr4fgp28o5vxodox1vocpfyo3w1.png)
As the value of equilibrium constant depends on rate constants of the forward and reverse reactions. And, this rate of reaction also changes with change in pressure and temperature.
Therefore, it will also lead to change in equilibrium constant but it does not depend on initial amount pf reactants.
Thus, we can conclude that in general, the value of the equilibrium constant for a chemical reaction does NOT depend on the initial amounts of reactants present.