Answer:
He
Step-by-step explanation:
According to Avogadro's law, at constant pressure and temperature, the volume of the gas is directly proportional to the moles of the gas.
Thus,
Moles of
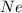 :-
:-
Mass of the gas = 22 g
Molar mass of Ne = 20.1797 g/mol
The formula for the calculation of moles is shown below:

Thus,
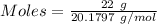
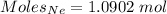
Moles of
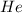 :-
:-
Mass of the gas = 22 g
Molar mass of He = 4.002602 g/mol
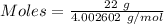
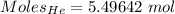
Moles of
 :-
:-
Mass of the gas = 22 g
Molar mass of
 = 31.998 g/mol
= 31.998 g/mol
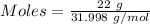

Moles of
 :-
:-
Mass of the gas = 22 g
Molar mass of
 = 70.906 g/mol
= 70.906 g/mol
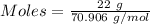
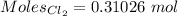
Thus, more the number of moles, more the volume. Thus, He will have the greatest volume at STP.