Answer:
Step-by-step explanation:
Light emitted by an atom making transition from higher energy state to low energy state has a frequency directly proportional to the energy change in electron.
Energy associated with atom is given by
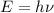
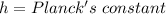
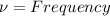
Change in Energy is