Answer:
350 J
Step-by-step explanation:
Q = Input heat of the steam engine = 2750 J
H = Heat energy given of to the surroundings = 1500 J
W = Work done by the system = 850 J
The energy which the system possesses is known as internal energy
Change in internal energy is given by
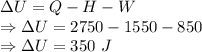
The change in internal energy of the system is 350 J