Answer:
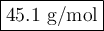
Step-by-step explanation:
Graham’s Law applies to the diffusion of gases:
The rate of diffusion (r) of a gas is inversely proportional to the square root of its molar mass (M).
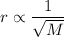
If you have two gases, the ratio of their rates of diffusion is
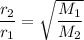
The time for diffusion is inversely proportional to the rate.
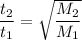
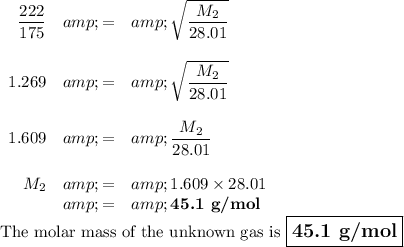
Data:
t₂ = 222 s
t₁ = 175 s
M₁ = 28.01
Calculation :