Answer:
The correct answer is option D.
Step-by-step explanation:
The chemical equation for the conversion follows:
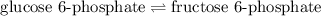
The expression for
 of above equation is:
of above equation is:
![K_(eq)=\frac{\text{[fructose 6-phosphate]}}{\text{[glucose 6-phosphate]}}](https://img.qammunity.org/2020/formulas/chemistry/high-school/u8ujw5dbaxd5uqs3pmpuay2pooyv3nkhpe.png)
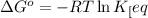
where,
 = standard Gibbs free energy = 1.72 kJ/mol = 1720 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 1.72 kJ/mol = 1720 J/mol (Conversion factor: 1kJ = 1000J)
R = Gas constant =
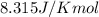 (given)
(given)
T = temperature =298K
Putting values in above equation, we get:
![1720 J/mol=-(8.315J/Kmol)* 298K* \ln (\frac{\text{[fructose 6-phosphate]}}{\text{[glucose 6-phosphate]}})](https://img.qammunity.org/2020/formulas/chemistry/high-school/v5zf1tcei5q7y04d90ljgymb3rea4qc0u7.png)
![\frac{\text{[fructose 6-phosphate]}}{\text{[glucose 6-phosphate]}}=0.499\approx = 0.5=(1)/(2)](https://img.qammunity.org/2020/formulas/chemistry/high-school/niibmjclcrmkgt6lp9in9az3eouzacos90.png)
![\frac{\text{[fructose 6-phosphate]}}{\text{[glucose 6-phosphate]}}=(1)/(2)](https://img.qammunity.org/2020/formulas/chemistry/high-school/pysejuev0jh5iujpnnrxrxzctdfxrqwrju.png)
The ratio of glucose 6-phosphate to fructose 6-phosphate at equilibrium :
![\frac{\text{[glucose 6-phosphate]}}{\text{[fructose 6-phosphate]}}=(2)/(1)](https://img.qammunity.org/2020/formulas/chemistry/high-school/uw6xn5da5wxp8hsgyyc6kz9477d07ffcw3.png)