Answer:
The Lewis structure shows a stable electronic structure.
Step-by-step explanation:
The Lewis structure given consists of Phosphorous (P) and Chlorine (Cl).
The atoms are considered to be stable if they have an octet configuration which means eight electrons in the valence shell.
The only exception is Helium (He) which is stable even though it has only two electrons in the valence shell.
All the atoms of the molecule are surrounded with eight electrons.
Hence, the given Lewis structure shows a stable electronic configuration.
The given molecule is
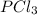 , Phosphorous Trichloride.
, Phosphorous Trichloride.