Answer:
The final temperature of the NaOH solution: T₂ = 37 °C
Step-by-step explanation:
Given: The enthalpy of NaOH solution:
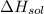 = – 44.4 kJ/mol,
= – 44.4 kJ/mol,
The specific heat capacity of solution: c = 4.184 J/g.K
Given mass of solute (NaOH): w = 13.9 g, Molar mass of NaOH: m = 40 g/mol
Mass of the solvent: W = 250 g
Initial temperature of the solution: T₁ = 23 °C = 23 + 273 = 296 K (∵ 0°C = 273 K)
Final temperature of the solution: T₂ = ?
The number of moles of NaOH =
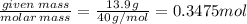
So the amount of heat released (ΔH) by dissolution of 13.9 g NaOH:
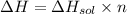
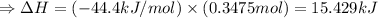
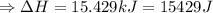
(∵ 1 kJ = 1000J )
Since the specific heat capacity is given by the equation:
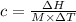
Here, M is the total mass of the solution = w + W = 13.9 g + 250 g =263.9 g
Thus the increase in the temperature (ΔT) can be calculated as:
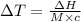
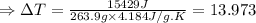
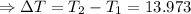
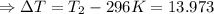
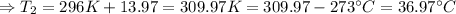
(∵ 0°C = 273 K)
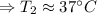
Therefore, the final temperature of the NaOH solution: T₂ = 37 °C