Answer:
The standard enthalpy change for the reaction at
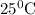 is -2043.999kJ
is -2043.999kJ
Step-by-step explanation:
Standard enthalpy change (
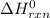 ) for the given reaction is expressed as:
) for the given reaction is expressed as:
![\Delta H_(rxn)^(0)=[3mol* \Delta H_(f)^(0)(CO_(2))_(g)]+[4mol* \Delta H_(f)^(0)(H_(2)O)_(g)]-[1mol* \Delta H_(f)^(0)(C_(3)H_(8))_(g)]-[5mol* \Delta H_(f)^(0)(O_(2))_(g)]](https://img.qammunity.org/2020/formulas/chemistry/college/p7w929gxrcymv1zwba8yaqlrsek6ritldc.png)
Where
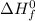 refers standard enthalpy of formation
refers standard enthalpy of formation
Plug in all the given values from literature in the above equation:
![\Delta H_(rxn)^(0)=[3mol* (-393.509kJ/mol)]+[4mol* (-241.818kJ/mol)]-[1mol* (-103.8kJ/mol)]-[5mol* (0kJ/mol)]=-2043.999kJ](https://img.qammunity.org/2020/formulas/chemistry/college/iwhttjbw27t3u2u317c5nx282doouf1jfz.png)