Answer:
11.32%
Step-by-step explanation:
Let's use as a calculus base 100 moles of the solute in water. If we call the number of moles of it that remains in water as x, the number of moles that is extracted in ether is 100 - x.
The partion coefficient is the concentration in the organic fraction (ether) divided by the concentration in water. And the concentration is the number of moles divided by the volume, thus:
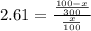
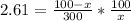
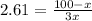
7.83x = 100 - x
8.83x = 100
x = 11.32 moles
Thus, the fraction that remains in water is the number of moles divided by the total multiplied by 100%
q = 11.32%