Answer:
4
Step-by-step explanation:
Relationship between wavenumber and Rydberg constant (R) is as follows:
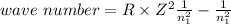
Here, Z is atomic number.
R=109677 cm^-1
Wavenumber is related with wavelength as follows:
wavenumber = 1/wavelength
wavelength = 253.4 nm
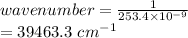
Z fro Be = 4
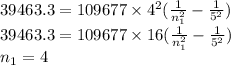
Therefore, the principal quantum number corresponding to the given emission is 4.