Answer:
-32821.2 J (negative sign implies that work is done by the system)
Step-by-step explanation:
The expression for the calculation of work done is shown below as:
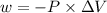
Where, P is the pressure
 is the change in volume
is the change in volume
From the question,
 = 18.0 - 1.00 L = 18.0 L
= 18.0 - 1.00 L = 18.0 L
P = 18.0 atm
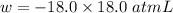
Also, 1 atmL = 101.3 J
So,
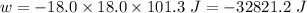 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)