Answer:
Final volumeof the gas = 2.84 L
Step-by-step explanation:
The formular to be used here is the general gas equation. the formular is being used because it gives the relationship between the three gas parameters (volume, temperature and pressure) mentioned.
The general gas equation is given as;
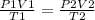
where;
P1 = initial pressure
V1 = initial volume
T1 = initial temperature
P2 = Final pressure
V2 = Final volume
T2 = Final temperature
From the question,
P1 = 1.00 atm
P2 = 0.85atm
T1 = 25C + 273 = 298K (Converting to kelvin)
T2 = 15C + 273 = 288K (Converting to kelvin)
V1 = 2.5L
V2 = ?
from the equation, making V2 subject of formula we have;
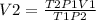
V2 = (1*2.5*288)/(298*0.85) = 2.84 L.