To solve this problem it is necessary to apply the relationships given between the number of moles, the atomic mass and the mass of the elements. In general, mass can be expressed in terms of these two terms as:
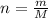
Where,
n = Number of mole
m = Mass
M = Molar mass
Since the mass quantity of the two elements is the same we will have the relation:
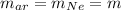
The number of mole of Argon is
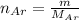
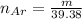
The number of moles of Neon gas is
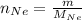
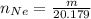
The number of moles of the gas mixture is
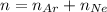
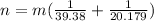
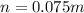
The percentage of the Neon is
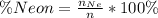
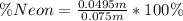
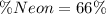
Therefore the percentage of Neon in the gas mixture is 66%