Answer:
The concentration of KOH is 0.186 M
Step-by-step explanation:
First things first, we need too write out the balanced equation between HBr and KOH.
This is given as;
KOH (aq) + HBr (aq) → KBr (aq) + H2O (l)
From the reaction above, we can tell that it takes 1 mole of KOH to react with 1 mole of HBr.
We use the acid base formular in calculating unknown concentrations. This is given as;
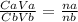
where;
Ca = Concentration of acid
Va = Volume of acid
Cb = Concentration of base
Vb = Volume of base
na = Number of moles of acid
nb = Number of moles of base
KOH is the base and HBr is acid.
Hence;
Ca = 0.225
Va = 35
Cb = ?
Vb = 42.3
na = 1
nb = 1
Making Cb subject of formular we have;
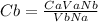
Cb = (0.225 * 35 * 1) / (42.3 * 1)
Cb = 0.186 M