Answer:
The solubility product of calcium hydroxide is
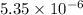 .
.
Step-by-step explanation:
To calculate the concentration of acid, we use the equation given y neutralization reaction:
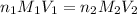
where,
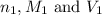 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is

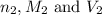 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is
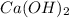 .
.
We are given:
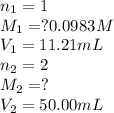
Putting values in above equation, we get:
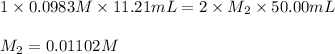
Molarity of calcium hydroxide solution = 0.01102 M
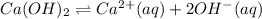
1 mole of calcium hydroxide gives 1 mole of calcium ions and 2 moles of hydroxide ions.
![[Ca^(2+)]=1* 0.01102 M=0.01102 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/rmw5vwszylz4k5bu73u5yli9nx16i12ni5.png)
![[OH^(-)]=2* 0.01102 M=0.02204 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/1cw45x6gqbj61qd0aw2s1jtyu2x43ec1g5.png)
Solubility product is given by:
![K_(sp)=[Ca^(2+)][OH^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/7nky2s7c80f0u39z9mkn8ij7t0zchfjo9y.png)
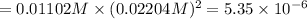
The solubility product of calcium hydroxide is
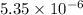 .
.