Answer:
Mole is a quantity corresponding to
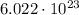 of units;
of units;
Difference in molar mass produces different masses for the same number of moles of two substances
Step-by-step explanation:
We can define a mole very easily. Mole may be considered in any system, not only in chemistry. However, mole is mostly significant in chemistry.
Mole is simply a unit of measurement which is equal to the Avogadro's number of objects. That is, if we have 1 mole of some specific object, it means we have a total of Avogadro's number (
![6.022\cdot 10^(23)) of objects.</p><p>For example, let's say that we have 2 moles of stones. This means we have a total of:</p><p>[tex]2\cdot N_A = 2\cdot 6.022\cdot 10^(23) = 1.2044\cdot 10^(24)]() stones.
stones.
Since this is a very huge number, we mainly use moles in chemistry to represent the number of molecules, atoms or ions.
You defined it perfectly: if we have 1 mol of C and 1 mol of Mg, they both contain the same number of atoms, that is, a total of
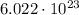 atoms.
atoms.
The problem is, however, that each individual atom of magnesium weighs more than the carbon atom. If you look at the periodic table, the molar mass of magnesium is 24.305 g/mol, while the molar mass of carbon is 12.011 g/mol. Magnesium atom is more than twice as heavy as carbon.
According to the molar mass, 1 mol of magnesium contains 24.305 grams and 1 mol of carbon contains 12.011 grams. This is essentially due to a different nature of atoms, they have different masses.
Take, for comparison, one stone and one feather. Although we have one of each, stones weigh more than feathers.